Connected Drug Delivery Devices Market Grows at 23.44% CAGR, Expected to Reach USD 61.08 billion by 2034
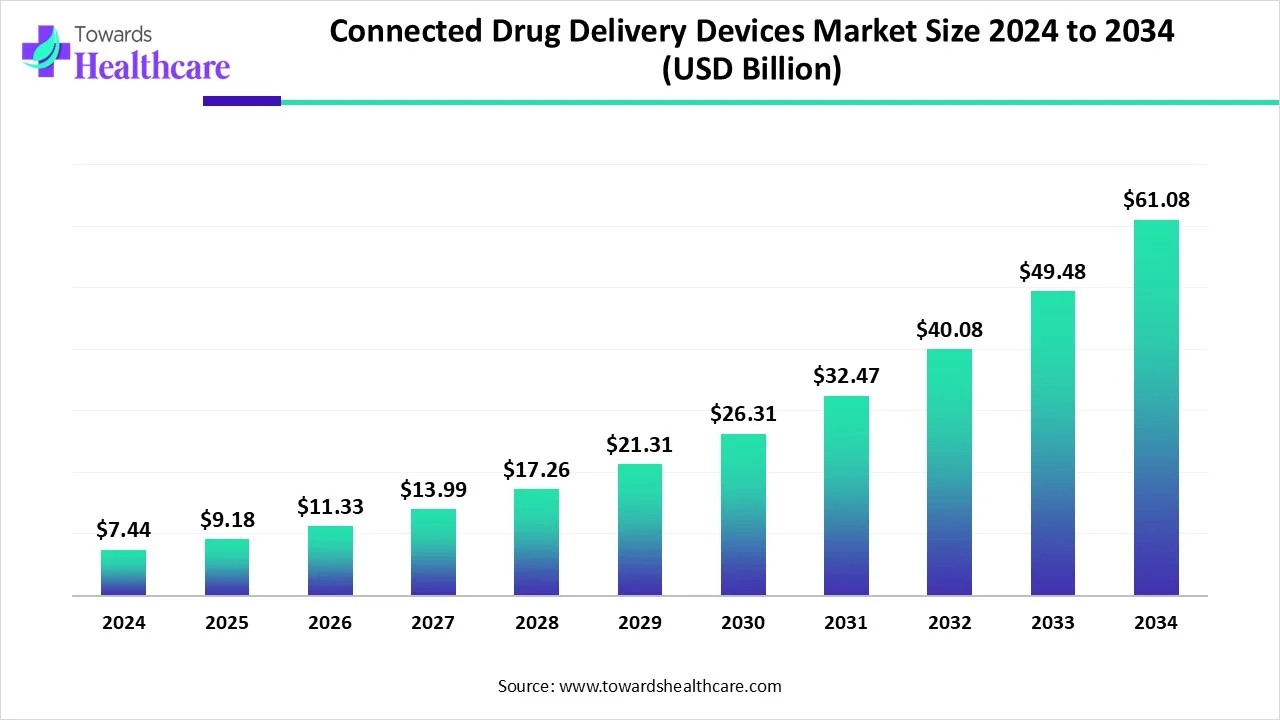
The connected drug delivery devices market size is calculated at USD 9.18 billion in 2025 and is expected to reach around USD 61.08 billion by 2034, growing at a CAGR of 23.44%.
Ottawa, Oct. 03, 2025 (GLOBE NEWSWIRE) -- The global connected drug delivery devices market size was valued at USD 7.44 billion in 2024 and is predicted to hit around USD 61.08 billion by 2034, rising at a 23.44% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research. This market is rising due to the escalating demand for smarter, connected therapeutics that improve medication adherence, remote patient monitoring, and real-time feedback in chronic disease management.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5736
Key Takeaways:
- Connected drug delivery devices sector is pushing the market to USD 7.44 billion in 2024.
- Long-term projections show USD 61.08 billion valuation by 2034.
- Growth is expected at a steady CAGR of 23.44% in between 2025 to 2034.
- North America held a major revenue share of the connected drug delivery devices market share by 36% in 2024.
- Asia-Pacific is expected to host the fastest-growing market in the coming years.
- By product type, the standalone components & software segment dominated the global market in 2024.
- By product type, the integrated devices segment is expected to expand at the fastest CAGR in the market in the coming years.
- By route of administration, the parenteral devices segment contributed the biggest revenue share of the market in 2024.
- By route of administration, the inhalation devices segment is expected to show the fastest CAGR over the forecast period.
- By application, the diabetes management segment registered its dominance over the global connected drug delivery devices market in 2024.
- By application, the COPD segment is expected to witness significant growth in the market over the studied years.
Market Overview:
The market for connected drug delivery devices is on track to become a sizable segment of digital health by merging drug delivery systems with sensors, wireless connection, and analytics to better connect patients, providers, and therapies. These devices will support automation of dosage timing, remote monitoring of patient use, and identification of patterns of adherence, which will help to enable the opportunity for individualized management of treatment.
There is increased impetus for devices that can support integration of pharmaceuticals with digital support given the current shift in health systems globally toward value-based care and patient-focused models. Developers are currently focusing on elements like modular architecture, interoperability, and secure data transfer, which will facilitate use in clinical systems. There is trend away from independent gadgets toward using connected platforms that spur on telehealth service.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Market Scope:
| Metric | Details | |
| Market Size in 2025 | USD 9.18 Billion | |
| Projected Market Size in 2034 | USD 61.08 Billion | |
| CAGR (2025 - 2034) | 23.44 | % |
| Leading Region | North America share 36% | |
| Market Segmentation | By Product Type, By Route of Administration, By Application, By Region | |
| Top Key Players | Aptar Digital Health, Becton, Dickinson and Company, Biocorp, Cohero Health, Inc., Medtronic, Novartis AG, Novo Nordisk, Propeller Health, SHL Medical, Tandem Diabetes Care, West Pharmaceutical Services, Ypsomed Holding | |
Major Growth Drivers:
- The escalating global burden of chronic diseases, such as diabetes, cardiovascular disease, respiratory illnesses, and autoimmune diseases means that patients will require more frequent and more precision dosing support, which connected devices can provide.
- Sensor and wireless technologies (Bluetooth Low Energy, NB-IoT, cellular networks, low-power WiFi) are constantly improving, leading to smaller, more efficient, and less expensive connectivity.
- Payers and health systems are increasingly valuing the adherence to medication, outcomes, and remote monitoring, resulting in reimbursement and incentive programs that explicitly support these connected devices.
- Increased adoption of telemedicine and remote care models (accelerated by the COVID-19 pandemic in 2020) has put pressure on providers to request devices that can integrate into virtual care health platforms.
- Regulatory bodies are moving toward more favorable consideration of digital medical devices and 'real world evidence,' simplifying pathways for approval and enabling partnership agreements between pharma and medtech.
Key Drifts:
- Manufacturers are creating multi-component architectures, including removable sensor devices or adapter kits that add connectivity to existing drug delivery assemblies or medical devices, vs. monolithic “smart injectors.”
- Many newer connected devices are embedding low level machine learning or rule-based logic on the device or near/ local device gateway while minimizing when devices send streaming raw data to the cloud. This enables alerts or detection of abnormal event while the device is not relying on streaming data to the network.
- More of the device vendors are developing APIs, SDKs, and standard compliance for integration into electronic health records, telehealth platforms, and patient apps. Partnerships between device vendors, software vendors, and health IT vendors are increasing to help streamline data flows.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Significant Challenge:
Data Privacy, Security and Regulatory Complexity:
Although connected drug delivery devices can be expected to generate frequent, rich data streams, they also introduce new significant risks related to data security, patient privacy and device/system integrity. Given the devices are dosing medications, any unauthorized access or attack/cyber breach to the devices could significantly impact patient safety. The regulatory landscape of medical devices that have digital/internet connected services is still evolving and varies immensely from one country to another, adding complexity to global commercialization.
Trials can take many forms and will not only demonstrate mechanical and pharmaceutical safety, but will also include considerations for cybersecurity, data encryption, firmware validation, and software upgrades. Payers and health systems may also hesitate to reimburse and adopt devices for those with unclear data governance and liability models. All of these factors will slow investment, increase time for a device to go through research and development, and add significantly to the entry bar for smaller firms.
Regional Analysis:
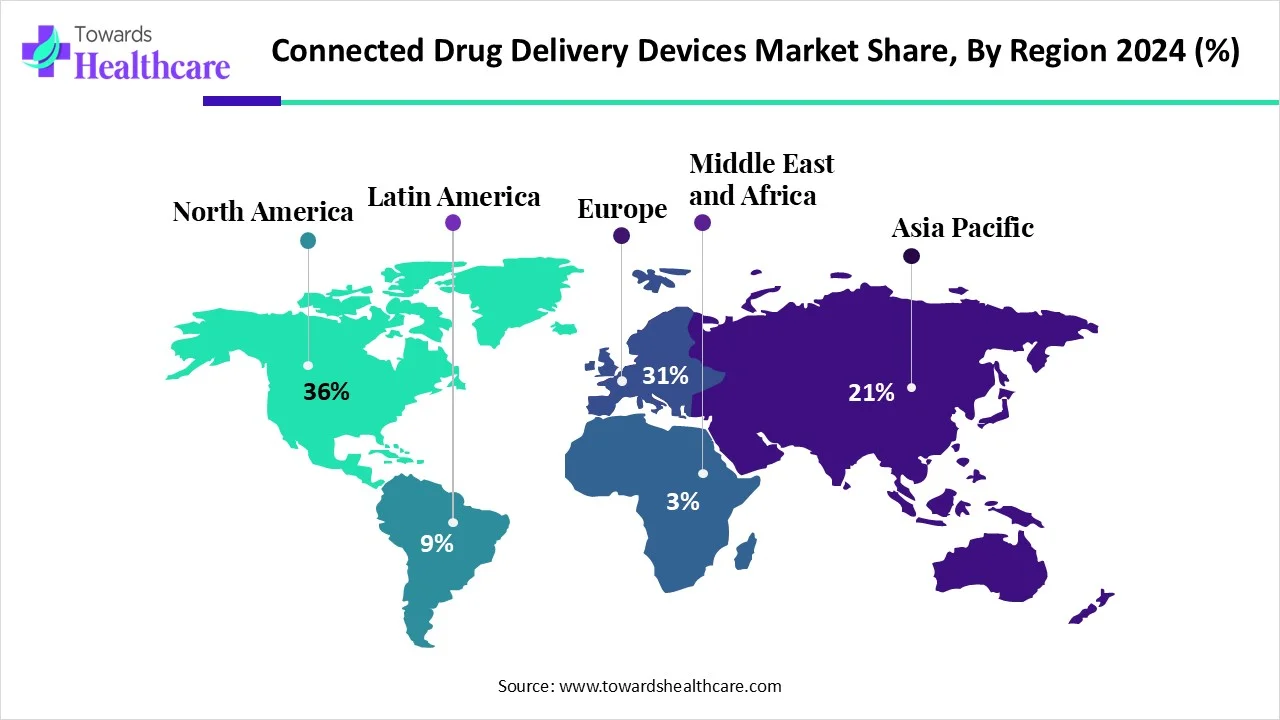
In the connected drug delivery devices industry, North America is the largest market share by 36% with more cases of chronic diseases, high healthcare expenditures, and strong uptake of digital health products. North America benefits from sophisticated infrastructure, clearly defined regulatory pathways, and a large population of pharmaceutical and medtech companies investing in connected technology. Favorable reimbursement models and integration with electronic health records bolster its competitive advantage.
Asia-Pacific is the fastest-growing market, with increasing cases of diseases like diabetes, asthma, chronic obstructive pulmonary disorder (COPD), and cardiovascular diseases across countries like China, India, and Japan. The impact of growing government support for telemedicine, rapid digitalization, and access to healthcare is driving growth in the connected drug delivery market in the region. Mobile connectivity along with increasing investments by start-ups and multinational companies is expanding access of connected drug delivery devices.
Download the single region market report @ https://www.towardshealthcare.com/price/5736
Asian Country-level Analysis:
- Digital Health Ecosystem Growth: Nations like China, India, South Korea, and Singapore are investing heavily in digital healthcare infrastructure. Governments are promoting smart hospitals and encouraging partnerships between medtech companies and AI startups, paving the way for device connectivity.
- Diabetes and Respiratory Disease Burden: With high rates of diabetes, asthma, and COPD, countries like India and China are creating demand for connected insulin pens, inhalers, and real-time monitoring tools that enhance medication adherence and disease management.
- Affordability & Localization: Local manufacturers in China and Southeast Asia are entering the connected device space with cost-effective smart injectors, add-on sensors, and Bluetooth-enabled inhalers, increasing accessibility across urban and semi-urban populations.
- Pharma Partnerships: Asian pharmaceutical companies are forming alliances with health tech firms to co-develop integrated drug + device + app ecosystems, especially in the fields of diabetes care and oncology.
-
Regulatory Modernization: Regulatory bodies in Japan, Singapore, and South Korea are streamlining digital health approvals, which is enabling faster commercialization of innovative drug delivery technologies.
Example: In India, the adoption of smart insulin pens is gaining ground as both public and private healthcare players look for ways to improve long-term diabetes management through tech-enabled adherence solutions.
Segmental Insights:
By Product Type:
Standalone components and software are currently the leading segment: sensor modules, firmware platforms, analytics engines, and connectivity middleware are core to the connected ecosystem. Several companies provide the term "smart modules" that can be affixed to standard injectors or inhalers, or provide back-end cloud/analytics.
On the other side of the product types are integrated devices, fully built drug delivery devices that embed connectivity from the ground up. Integrated devices are the overall fastest growing segment, as manufacturers are trending toward all-in-one intelligent injectors or inhalers.
Get the latest insights on healthcare industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By Route of Administration:
Within routes of delivery, the parenteral segment (injectables, infusions) remains the largest route of delivery as many chronic therapies (e.g., insulin products, biologics) are delivered by injection and have the greatest potential for monitoring and adherence support based on their chronic nature.
The inhalation segment is the fastest growing route of delivery as respiratory therapies (COPD, asthma) are integrating connectivity to monitor the use of the inhaler, medication delivery technique, and obtain a longitudinal assessment of their inhaler use.
By Application:
As far as application, diabetes dominates with connected drug delivery through insulin pens and pumps is driving adoption as dosing is frequent and adherence is crucial; smart pen sensors and dose-tracking applications support adherence for patients and providers/tracers.
COPD is the fastest growing segment with the emergence of connected inhalers and feedback systems that enable the patient to know when their inhalation technique is correct and is promoting adherence and chronic disease monitoring in respiratory patients.
Recent Developments:
In November 2024, Medtronic obtained FDA clearance for the new InPen smart insulin pen app, which works with its Simplera continuous glucose monitor (CGM). This clearance makes possible the "Smart MDI" system that can recommend corrections for missed or inaccurate insulin doses at mealtimes.
Browse More Insights of Towards Healthcare:
The global arthroscopy devices market was valued at USD 25.03 billion in 2024, rising to USD 27.16 billion in 2025, and is projected to reach nearly USD 56.75 billion by 2034, advancing at a CAGR of 8.53% between 2024 and 2034.
The drug-device combination products market stood at USD 150.3 billion in 2023 and is expected to grow steadily, reaching about USD 337.81 billion by 2034, expanding at a CAGR of 7.64% over the forecast period (2024–2034).
The nebulizer devices market was valued at USD 1.17 billion in 2023 and is anticipated to climb to around USD 2.2 billion by 2034, reflecting a CAGR of 5.94% during 2024–2034.
The medical device gaskets and seals market generated USD 0.92 billion in 2023 and is set to reach nearly USD 1.57 billion by 2034, growing at a CAGR of 5% from 2024 to 2034.
The ventricular assist devices (VAD) market is projected to increase from USD 1.82 billion in 2025 to about USD 3.38 billion by 2034, with a CAGR of 7.1% over the forecast period.
The 3D-printed medical devices market is expected to expand rapidly, from USD 5.59 billion in 2025 to nearly USD 24.69 billion by 2034, registering a strong CAGR of 17.94%, driven by rising demand for personalized healthcare solutions.
The respiratory devices market is forecast to grow from USD 26.57 billion in 2025 to approximately USD 56.48 billion by 2034, progressing at a CAGR of 8.74%.
The anesthesia monitoring devices market is projected to more than double, rising from USD 3.17 billion in 2025 to USD 7.16 billion by 2034, with a CAGR of 9.5%.
The blood pressure monitoring devices market was valued at USD 2.30 billion in 2024 and is forecast to reach nearly USD 8.62 billion by 2034, growing at a robust CAGR of 14.1%.
The blood pressure measuring devices market surpassed USD 1.75 billion in 2024, is estimated at USD 1.91 billion in 2025, and is projected to expand to USD 4.18 billion by 2034, at a CAGR of 9.1%.
Key Players in Connected Drug Delivery Devices Market:
Aptar Digital Health
Offers connected solutions for respiratory and injectable drug delivery, integrating sensors and software to enhance treatment adherence and outcomes.
Becton, Dickinson and Company
Develops smart injection systems and connected autoinjectors focused on improving medication delivery accuracy and patient monitoring.
Biocorp
Specializes in connected devices like smart caps and add-ons for injectors, enabling real-time data tracking and dose adherence.
Cohero Health, Inc.
Provides connected respiratory devices and digital platforms for managing asthma and COPD through medication tracking and lung function monitoring.
Medtronic
Delivers advanced connected insulin pumps and continuous glucose monitoring systems to support diabetes management.
Novartis AG
Integrates digital health solutions with inhalation therapies, focusing on connected respiratory drug delivery to improve adherence.
Novo Nordisk
Offers smart insulin pens and digital diabetes tools designed to monitor dosing and sync with mobile health apps for personalized care.
Propeller Health
Develops digital inhaler sensors and companion apps that track usage patterns and provide real-time feedback to patients and clinicians.
SHL Medical
Produces connected autoinjectors and wearable drug delivery systems, integrating Bluetooth and app-based technologies for chronic care.
Tandem Diabetes Care
Manufactures connected insulin pumps with advanced algorithms and wireless capabilities for real-time diabetes management.
West Pharmaceutical Services
Offers connected containment and delivery systems, including smart wearable injectors and integrated drug-device platforms.
Ypsomed Holding
Provides connected pen injectors and autoinjectors with digital interfaces, supporting data capture and integration with digital health platforms.
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/price/5736
Segments Covered in the Report
By Product Type
- Standalone Components & Software
- Integrated Devices
By Route of Administration
- Parenteral
- Inhalation
By Application
- Diabetes Management
- Asthma
- COPD
- Others
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/price/5736
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

